Description
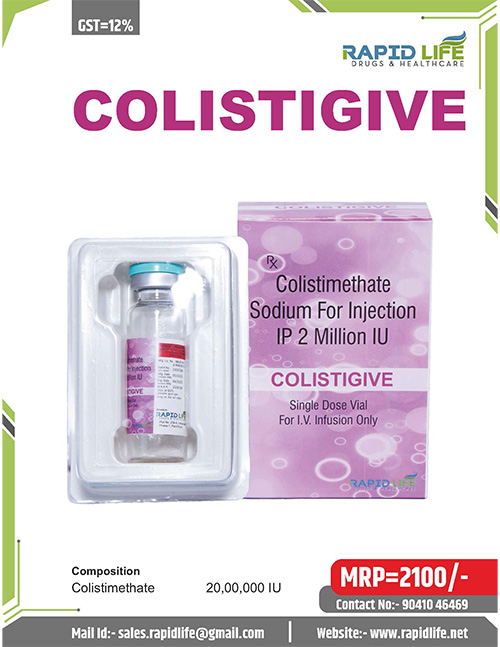
Colistigive is a high-end Colistimethate Injection formulated by Rapid Life Drugs and Healthcare to counter the increased insidiousness of bacterial infections that are severe and resistant to drugs. All the vials include Colistimethate Sodium (Colistin 2 Million IU) 2 MIU, which is a representative of the Polymyxin line of antibiotics. Colistigive is prepared to high standards that match those of hospitals and ICUs that have a high degree of consistency in terms of potency, stability, and safety of patients. Rapid Life Drugs & Healthcare, as a responsible pharmaceutical manufacturing, supplier, and distributor, will ensure the reliability of the supply of Colistigive to tertiary care hospitals, critical care units, and other healthcare facilities.
Composition:
Each vial contains: Colistimethate Sodium equivalent to Colistin 2 million IU (2 MIU)
Therapeutic Class: Polymyxin Antibiotic
(Indicated for the treatment of severe gram-negative bacterial infections, including multidrug-resistant Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae)
Medical Uses
Colistigive is advised to use in severe Gram-negative infections, especially in cases of multidrug-resistant organisms. It is the first-line treatment for infections caused by Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae, where other antibiotics cannot help. Colistigive is one of the main drugs used for the management of hospital-acquired pneumonia, infections due to ventilators, sepsis, and difficult urinary tract infections in patients with a critical condition.
Directions to Use
Colistigive demands medical attention only when used. The solution is reconstituted and administered by intravenous administration as advised by clinical provisions. The doses, the frequency, and the duration of therapy depend on the severity of infection, the weight of the patient, the performance of his kidneys, and the general state of health. Renal-impaired patients require dose compensation to reduce toxicity and ensure safe therapy.
Precautions
Close observation is needed in Colistigive therapy, especially on renal condition and neurological status. It is not to be administered carelessly in patients with renal loss, neuromotor disorders, or those taking any other nephrotoxic agents. They must avoid cases of self-medication or premature termination to avoid failed treatment and resistance.
Possible Side Effects
There may be occurrences of nephrotoxicity, neurotoxicity, dizziness, tingling sensations, muscle weakness, or injection-site reactions. The vast majority of side effects are reversible and dose-dependent with the help of timely medical intervention. Emerging symptoms are to be referred to as soon as possible.
Frequently Asked Questions
Q: What is the main purpose of Colistigive?
A: Colistigive treats severe infections by gram-negative multidrug-resistant bacteria when the standard antibiotics have failed.
Q: Who is the manufacturer of Colistigive injection?
A: Colistigive is produced by Rapid Life Drugs and Healthcare, which deals with critical care medicines of a higher quality.
Q: Can Colistigive be used in the ICU patients?
A: Yes, Colistigive is a common drug in the ICUs in the treatment of life-threatening resistant bacterial infections.
Q: Is Colistigive under renal monitoring?
A: Yes, the kidney monitoring is a vital part of the therapy to be considered safe and to make necessary alterations in the dose.
Q: Is it possible to use Colistigive as the first-line treatment?
A: Colistigive is generally only used as the last option for resistant infections after the failure of other antibiotics.
unsuitable.
Q: Does Colistigive work when administered correctly?
A: It is safe and effective when used carefully with careful monitoring, especially with serious infections.
Q: Does Colistigive come from authorized distributors?
A: Yes, Rapid Life Drugs & Healthcare is the distributor of Colistigive, using its effective drug distribution network.