Description
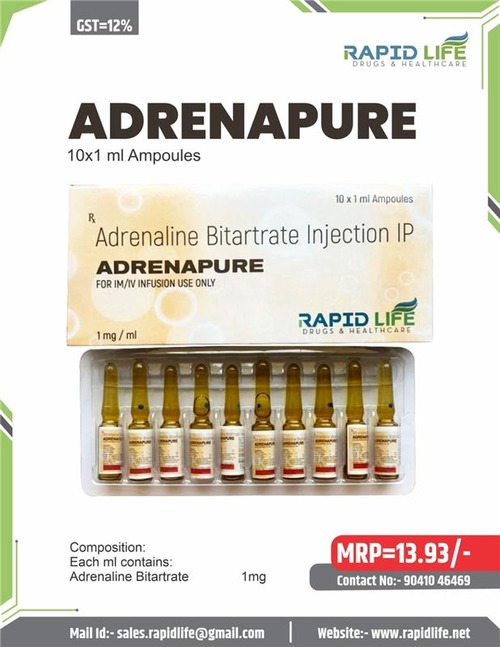
Composition:
Each ml contains:
-
Adrenaline Bitartrate equivalent to Adrenaline 1 mg
Therapeutic Class: Sympathomimetic Agent (Adrenergic Agonist)
(Indicated for emergency treatment of anaphylaxis, cardiac arrest, and severe acute allergic reactions)
Adrenaline bitartrate Injection is a direct-acting adrenergic bronchodilator, recommended for the management of open-angle glaucoma, acute asthma, anaphylactic shock, as well as early cardiac life support.
The action and process of Adrenaline bitartrate injection stimulates sympathomimetic receptors (alpha and beta), whose action brings about relaxation of smooth and cardiac muscular tissues and dilation of skeletal muscles. The drug inhibits the secretion of liquid humour and stimulates outflow. The medication dilates pupils through the muscular contraction of dilator muscles.
Dosing Of Adrenaline Bitartrate Injection
- The dosages of the injection can be retaken in every 10-15 minutes, in accordance with the requirement.
- Give 0.15 mg of 1:2000 intermixture as Adrenaline bitartrate Injection
- For the management of chronic asthmatic attack (above 4 years): 160- 250 mcg metered aerosol is equivalent to one inhalation. The dose can be repeated after 1 minute.
- For fixation of cardiac beat in cardiac arrest patients (Adult): Administer 0.5 to 1.0 mg of Adrenaline bitartrate Injection. The dose can be repeated every 3-5 minutes.
Benefits Of Adrenaline Bitartrate Injection
- Adrenaline bitartrate Injection is used to quickly alleviate life-threatening allergic reactions to medications or other agents provoking allergies.
- It is a first-line injection given during cardiopulmonary resuscitation (CPR) for the reversal of cardiac arrest.
- It is also used in the emergency treatment of shock caused by a fatal allergic reaction.
- Also, it is used to help surgeons during surgery by causing and maintaining mydriasis (pupil dilation) during ocular surgery.
Precautions
Adrenaline bitartrate Injection is not advisable in patients hypersensitive to sulphite compounds, patients suffering from angle-closure glaucoma and CVD patients. Care should also be taken in the use of BPH, patients with hypertension, and diabetic patients.
- It is not to be prescribed within 14 days of MAOI therapy.
- The drug can interact with ergot alkaloids, antihistamines, alpha-adrenergic blockers, beta-adrenergic blockers, cardiac glycosides, L-dopa, TCA, thyroid hormones, general anaesthetic medicines, diuretics and guanethidine.
- USFDA pregnancy category C. Might be or might not be deleterious to an unborn infant. Consult with the physician if you are pregnant or preparing to give birth while managing Adrenaline bitartrate injection.
- Whether the mediate traverse through breast milk or not is not well recognised. Lactating female parents should avoid breastfeeding when taking this drug.
- Adrenaline bitartrate must not be placed ‘in action’ with children unless recommended by a paediatrician.
Frequently Asked Questions ( FAQ )
How soon does this injection act?
The action of this injection can be seen after one hour of use.
How long do the effects of this injection last?
The action of this drug lasts for a mean time of 4-5 hours.
Is it safe to drink alcohol while on this injection?
Consult your physician before taking this medicine.
Is this a habit-forming medicine?
There were no habit-forming tendencies reported.
Can this medicine be used during pregnancy?
This medication is not indicated for pregnant women unless necessary. All the risks and benefits should be weighed with the doctor prior to using this medicine.
Can this medicine be used while breastfeeding?
This injection is not recommended in breastfeeding women only if essential. All the advantages and disadvantages should be explained to the physician before taking this injection .






Reviews
There are no reviews yet.